Adsorption and reaction
of dibromobenzene at the at the Si (111) 7x7 surface
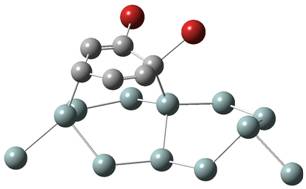
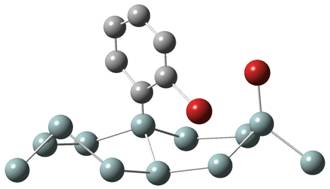
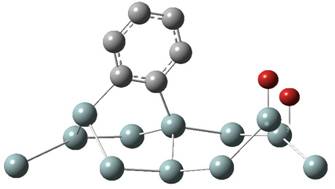
1. Chemisorption 2. Br-atom transfer 3. Second Br-atom transfer
Reaction
between 1,2-dibromobenzene and the Si(111)-7 [1]
7 surface has been studied theoretically on the
DFT(B3LYP/6-31G(d))
level. A 12-atom silicon cluster, representing two adatoms and one rest atom of
the
faulted half
of the unit cell, was used to model the silicon surface. The first step of the
reaction was a covalent
attachment
(chemisorption) of an intact 1,2-dibromobenzene molecule to the silicon
cluster. Binding energies
were
calculated to be between 1.04 and 1.14 eV, depending on the orientation of the
molecule. A second step
of the
reaction was the transfer of the Br atom to the silicon cluster. Activation
energies for the transfer of
the Br atom
were calculated to be between 0.4 and 0.6 eV, suggesting that the thermal
bromination reaction
occurs on a
microsecond time scale at room temperature. A third step of the reaction could
be the transfer of
the second
Br atom of the molecule, the desorption of the organic radical, or the change
of the adsorption
configuration
of the radical, depending on the original orientation of the adsorbed intact
molecule. A novel,
aromatic,
two-σ-bound adsorbed configuration of the C6H4
radical, in which a carbon ring of the radical is
perpendicular
to the silicon surface, has been introduced to explain previous experimental
observations (Surf.
Sci. 2004,
561, 11).
This work was published in: “An STM study of the localized atomic reaction of 1,2- and 1,4-dibromobenzene at Si (1 1 1)-7 × 7”, S. Dobrin, K.R, Harikumar and J.C. Polanyi, Surface Science, 561 (2004) 11;
"Reaction of 1,2-Dibromobenzene with the Si(111)-7×7 Surface, a DFT Study", S. Dobrin, J. Phys. Chem. B. 109 (2005) 22976