Photoinduced Charge
Transfer on sodium clusters and
Rydberg H-atom Time
of Flight spectroscopy of the products
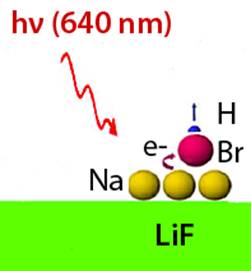 Laser irradiation induces charge transfer between sodium and HBr molecules (or HCl, HF).
Laser irradiation induces charge transfer between sodium and HBr molecules (or HCl, HF).
The
newly formed HBr - anion is unstable and undergoes
dissociation to Br and H-atoms.
The
Br‑atom remains on the surface and forms a
Na-Br bond, while the H-atom is ejected into the gas phase.
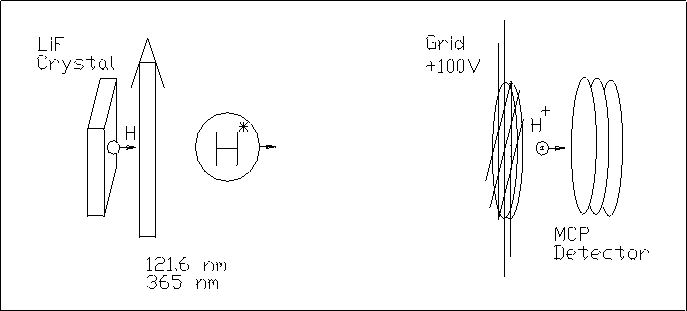
In the Rydberg H-atom Time of Flight spectroscopy experiment, the
desorbed H atoms are detected through the high lying
Rydberg states. This detection method is more
sensitive than the usual quadrupole mass spectrometry and allows one to detect
very small hydrogen atom fluxes from the surface.
The detection is done in the following way: after the hydrogen atom
desorbs from the surface, it passes through two laser beams. One laser beam
(121.6 nm) excites the H atom to the first electronically excited state. The
121.6 nm wavelength is formed by the tripling of 364.8 nm radiation of a dye
laser in the Ar/Kr gas cell. A second laser (around
365 nm) beam excites the hydrogen atom to the high-lying Rydberg state. Then,
the Rydberg H-atom comes to the microchannel plate located ~50 cm far from the
surface. In front of the microchannel plate, the Rydberg H-atom passes through
a 100 V biased grid, where it is ionized by an electric field and the resulted
cation H+ is detected by the microchannel plate.
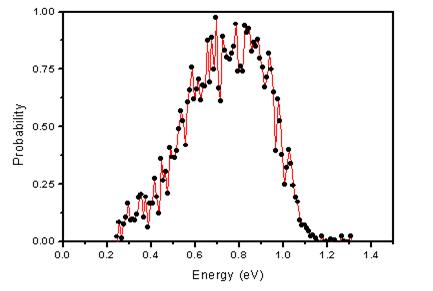
The time of flight spectrum of desorbed H‑atoms shows a structure in the translational energy
distribution. This structure is due to the vibrational excitation of the NaBr particles residing on the surface.
Published in: S. Dobrin, J. B. Giorgi, H. He, F. Y. Naumkin,
J.C. Polanyi, and S.A. Raspopov. J. Chem. Phys.,
119, 9795 (2003);
S. Dobrin, J. B, F.Y. Naumkin, and J.C. Polanyi, J. Chem. Phys., 122,
paper #014705 (2005)